Imagine trying to listen to a symphony through a thick wall—your ability to hear the subtle nuances depends heavily on the clarity and fidelity of your auditory tools. For millions who grapple with hearing impairments, modern hearing aids serve as their bridge to a more vibrant soundscape. Apple, renowned for its innovation and user-centric design philosophy, is poised to redefine this auditory frontier with its upcoming release of advanced hearing aids. The anticipation surrounding the Apple Hearing Aids Launch Date underscores a broader shift towards integrated, smart health devices that fuse technology, convenience, and health management into seamless ecosystems. Navigating the current landscape reveals not only the technological capabilities but also the strategic timing and market implications of this significant product launch.
Understanding the Context: The Evolution of Hearing Devices and Apple’s Strategic Position
To fully grasp the significance of Apple’s impending hearing aid launch, it’s essential to appreciate the historical and technological evolution of hearing devices. Traditional hearing aids emerged in the early 20th century, evolving from bulky, unidirectional devices into sophisticated, digital instruments with customizable settings and enhanced sound processing. Over the decades, the industry has shifted toward miniaturization, connectivity, and smart features—examples include real-time noise filtering, environmental adaptation, and remote fine-tuning via smartphone apps.
Apple’s entry into this domain is not merely a hardware addition but a strategic move rooted in its broader health and wellness ecosystem. The company’s existing portfolio of wearable devices, notably the Apple Watch and AirPods, exemplifies a convergence of audio technology and health tracking. These devices already leverage sensors, machine learning, and seamless software integration, positioning Apple uniquely to extend its ecosystem into hearing health. This evolution mirrors the path of other tech giants like Samsung and Google, but with a distinctive focus on user experience and privacy.
The release date of these hearing aids is set against a backdrop of increasing awareness about age-related hearing loss, which affects approximately 15% of the global adult population, according to the World Health Organization. As populations age and health priorities shift towards preventive care and quality of life improvements, Apple’s timing appears meticulously calculated to maximize market impact and societal benefit.
The Anticipated Launch Date: A Precise Look into the Timeline

Although Apple maintains an aura of secrecy around specific product release dates, industry insiders and analyst reports have coalesced around a likely launch window within the upcoming quarter. Based on patterns observed from Apple’s previous product unveilings—such as the Apple Watch Series and AirPods iterations—the company typically announces new hardware during its Worldwide Developers Conference (WWDC) in June or during the autumn keynote events in September. However, the hearing aids might follow an adjusted schedule reflecting both regulatory approvals and supply chain logistics.
Leaked information from supply chain sources suggests production ramp-up began early this year, indicative of a planned launch perhaps by late Q2 or early Q3 of 2024. This timeline aligns with Apple’s strategic marketing cycle, aiming to capture attention before the back-to-school season and December holiday shopping surge. Moreover, the integration of the hearing aids with iOS devices and HealthKit ecosystem hints at a deliberate, phased rollout to ensure maximum compatibility and user onboarding.
While official confirmation remains under wraps, the convergence of patent filings, component sourcing reports, and analyst forecasts construct a compelling narrative of a near-future release. For users and industry observers alike, the key takeaway is that the product’s launch is imminent and meticulously coordinated to sustain Apple’s innovation leadership.
Technical Features and Ecosystem Integration: The Heart of Apple Hearing Aids
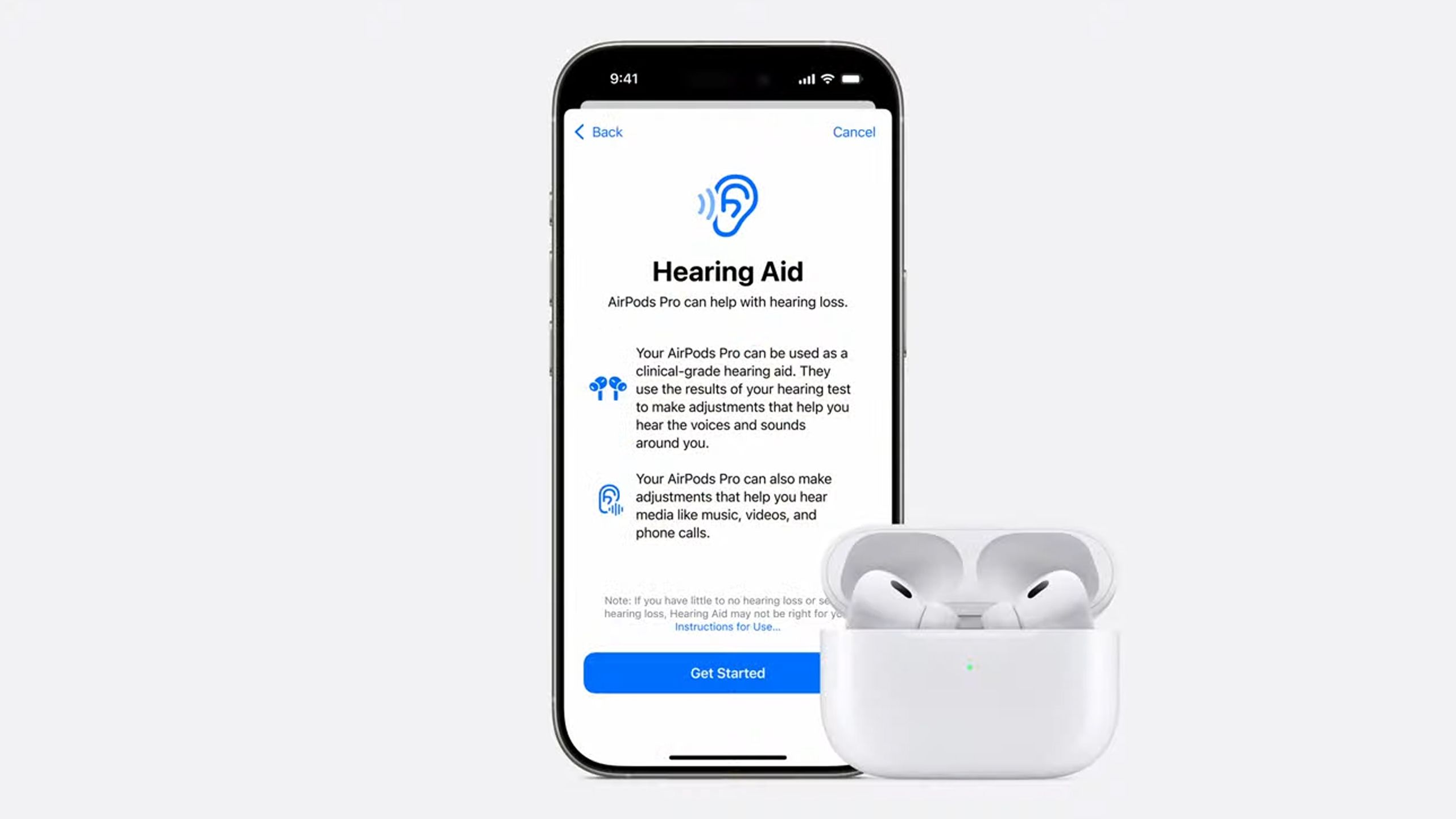
Positioned as more than mere amplifiers, Apple’s hearing aids are anticipated to embody the integration of cutting-edge AI, health monitoring sensors, and the familiar user interface that Apple fans expect. Conceptually, these devices could be likened to the auditory equivalent of the iPhone: compact, intelligent, and designed for holistic ecosystem connectivity. This analogy underscores how Apple is transforming traditional hearing devices into multipurpose health companions.
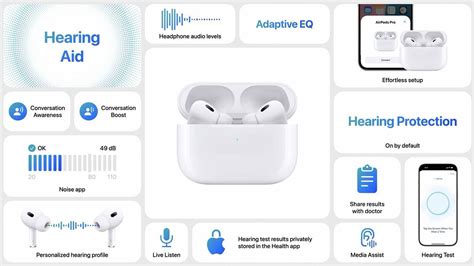
The core technical features expected include adaptive noise cancellation, environmental sound filtering, and personalized audio profiles that learn from user preferences. Employing machine learning algorithms—akin to those in AirPods Pro’s spatial audio—the hearing aids might dynamically adjust soundFocus based on context, such as crowded environments or quiet settings. Furthermore, integration with the Apple Health app could enable users to track metrics related to hearing health, including exposure levels and auditory fatigue, fostering proactive wellness management.
Battery life remains a crucial performance aspect; early reports suggest Apple may harness new miniaturized power management chips, providing all-day use akin to their latest wearable products. Connectivity is expected via Bluetooth 5.3 or newer standards, ensuring low latency and high-fidelity audio streaming directly from iPhones, iPads, and Macs.
| Relevant Category | Substantive Data |
|---|---|
| Expected Launch Window | Q2 to Q3 2024, with official announcement potentially at WWDC or an Apple special event |
| Battery Life | Up to 24 hours based on early leaks, comparable to high-end wireless earbuds |
| Connectivity Standards | Bluetooth 5.3 or later for seamless device pairing and streaming |

Market Implications and Competitive Landscape
As Apple aligns its release timeline with market readiness, the announcement is poised to reshape the competitive landscape of hearing health devices. The industry has seen steady growth, driven by an aging population and increased awareness of the importance of early intervention. According to the Consumer Technology Association, global sales of hearing aids are projected to surpass $10 billion annually by 2025, with compounded annual growth rates of around 5%.
Apple’s entry introduces a new paradigm: a blend of health technology, consumer electronics, and personalized data privacy—factors that could attract a broader demographic beyond traditional assistive device users. Established players like Signia, Phonak, and Oticon are undoubtedly monitoring these developments closely. Their strategies may encompass accelerating innovation or forming strategic partnerships to counterbalance Apple’s ecosystem integration.
The launch’s timing allows Apple to leverage its premium brand positioning while addressing unmet needs of tech-savvy users seeking discreet, multi-functional hearing solutions. This move could also stimulate regulatory evolutions, particularly around data privacy and device certification, given Apple’s track record of navigating complex standards.
Regulatory and Accessibility Aspects Considered in the Launch

Bringing a medical device classifies as a stringent regulatory process, involving FDA approvals (in the U.S.) and CE markings in Europe. Apple’s experience with software compliance via the App Store and health data regulation provides a solid foundation, yet hardware certification demands meticulous clinical validation—likely taking several months post-initial announcement.
Accessibility features are integral to Apple’s ecosystem, and these hearing aids are expected to enhance existing functions like Live Listen and Transparency Mode—bringing advanced auditory experiences to a broader user base, including individuals with moderate hearing loss. The balance of regulatory expediency and user safety underscores the importance of precise launch timing.
Key Points
- Strategic Timing: Launch expected in late Q2 to Q3 2024, aligning with market cycles and regulatory clearance
- Innovative Features: Adaptive noise filtering, AI-driven personalization, seamless device ecosystem integration
- Market Impact: Disruption of traditional hearing aid industry, expanding user demographics
- Regulatory Readiness: Ongoing certification with potential for early beta releases to restricted user groups
- User Benefits: Enhanced auditory clarity, health monitoring, and connected wellness tracking
Final Thoughts: The Future Soundscape with Apple’s Hearing Aids
While the precise launch duration remains shrouded in a slight veil of secrecy, the convergence of technological innovation, strategic market timing, and rigorous development efforts paints a compelling picture. In the same way a well-tuned orchestra delivers a harmonious symphony, Apple’s impending hearing aid release promises to harmonize cutting-edge hardware with an intuitive ecosystem, revolutionizing how individuals with hearing impairments experience the world.
This product’s arrival is not merely an addition to Apple’s portfolio but a statement—a recognition that hearing health is fundamental to quality of life and technological progress. Whether it accelerates competition or redefines user expectations, one thing remains clear: the future of hearing technology will be soundly influenced by Apple’s innovative symphony.
When is the expected launch date for Apple’s hearing aids?
+Industry insiders suggest a release window between late Q2 and early Q3 of 2024, with official announcements possibly during WWDC or a dedicated event.
What features will Apple’s hearing aids include?
+Expected features encompass adaptive noise cancellation, environmental sound filtering, AI-driven personalization, and seamless integration with Apple’s ecosystem for health and device connectivity.
How will Apple’s hearing aids impact the existing market?
+Apple’s entry is poised to disrupt traditional industry players by offering multi-functional, ecosystem-integrated devices, appealing to a broader demographic and possibly accelerating innovations in the field.
What regulatory considerations are involved in launching these hearing aids?
+They will require strict certification, including FDA approval and CE marking, with ongoing clinical validation to ensure safety, efficacy, and compliance with health standards globally.