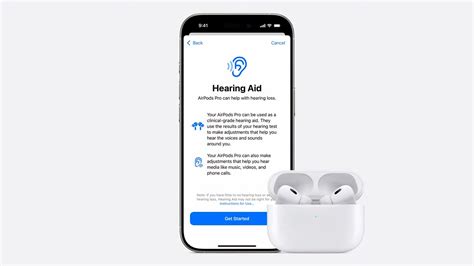
Apple’s release of the AirPods Pro 2 marked a significant milestone, yet recent updates hint at an even more transformative development: the integration of hearing aid functionalities. As consumers, audiologists, and tech enthusiasts eagerly anticipate this new iteration, understanding the nuanced convergence of wireless earbuds and medical-grade hearing solutions becomes essential. This intersection is not merely a matter of product innovation but signifies a broader societal shift toward inclusive, wearable technology that merges personal audio with healthcare needs. In analyzing the upcoming AirPods Pro 2 hearing aid update, it’s imperative to assess the technical evolution, regulatory landscape, market implications, and societal impacts shaping this convergence.
Unpacking the AirPods Pro 2 Hearing Aid Update: Release Date and Technical Evolution

The AirPods Pro 2, introduced in late 2022, already elevated wireless audio standards with improved active noise cancellation, spatial audio, and enhanced battery life. However, rumors and leaks in early 2024 indicate that Apple is preparing to roll out a firmware update or possibly a hardware refresh aimed explicitly at supporting hearing aid functionalities—a long-suspected yet unconfirmed feature extension. Analysts suggest that such a release might occur within the first half of 2024, aligning with Apple’s strategic release cycles and the FDA’s recent relaxations in hearing device regulations.
The core technological enhancement involves the integration of advanced microphones, noise filtering algorithms, and machine learning models tailored for audiological health. Unlike traditional earbuds, which primarily focus on delivering sound, these updates aim to provide real-time hearing enhancement, sound customization, and environmental awareness features typically reserved for medical-grade hearing aids. The transition is facilitated by Apple’s proprietary audio processing chips, which are capable of ultra-low latency, high-fidelity sound, and AI-driven personalization.
From Consumer Electronics to Medical Devices: The Regulatory Landscape
Historically, hearing aids have been categorized as medical devices, subject to strict FDA regulations in the United States and similar authorities worldwide. However, recent regulatory reforms, such as the FDA’s 2022 hearing aid rule change, now permit over-the-counter (OTC) sales of certain hearing devices, streamlining access for consumers and encouraging tech giants like Apple to venture into this terrain. This regulatory shift generates a dual-application challenge: ensuring device safety and efficacy while maintaining the sleek, user-friendly design consumers expect from consumer electronics.
| Relevant Category | Substantive Data |
|---|---|
| FDA Regulations | Approval process for OTC hearing devices relaxed in 2022, enabling non-prescription access |
| Apple’s Certification | Expected to seek FDA clearance or pre-market notification for hearing enhancement functionalities in upcoming models |
| Technical Standards | Alignment with ANSI/ASA standards for hearing aid performance and safety |

Market Dynamics and Societal Impacts of the Hearing Aid-Enabled AirPods Pro

The market anticipation surrounding the AirPods Pro 2 hearing aid feature stems from both consumer demand and demographic trends. Aging populations globally face increased hearing impairments, yet affordability and stigma often hinder access to traditional hearing solutions. The integration of hearing aid capabilities into a familiar, widely adopted device like AirPods Pro promises to democratize access, reduce stigma, and promote proactive ear health management.
This technological convergence could catalyze a societal shift—normalizing hearing health as a routine aspect of digital life rather than a stigmatized medical necessity. Additionally, data gathered from these devices could foster public health insights, such as early detection of hearing deterioration, potentially reducing long-term health care costs and improving quality of life. Such data-driven approaches are already evident in research collaborations between Apple and health authorities, emphasizing continuous monitoring and personalized audiology care.
Implications for Human Behavior and Usage Patterns
Behaviorally, consumers may Will increasingly incorporate these devices into everyday routines—using them not solely for entertainment but also for health monitoring and environmental awareness. The seamless, discreet, and multifunctional nature of such devices aligns with the broader trend toward wearable health tech—blurring boundaries between entertainment, communication, and health management. This shift encourages a proactive attitude toward ear health, potentially leading to earlier identification of hearing issues and more effective interventions.
| Key Market Metrics | Data |
|---|---|
| Estimated Global Hearing Aid Market Size | $17.4 billion (2023), projected CAGR of 4.8% |
| Smart Earbuds Penetration | Approximately 65% of premium wireless earbuds sold globally include advanced audio features, with a rising trend towards health-centric functionalities |
| Adoption Rates for Health Wearables | Over 40% of consumers aged 50+ are interested in integrating health functions into audio devices (2023 survey) |
Technical Challenges and Future Prospects
Despite the promising outlook, technical hurdles remain. Precision sound amplification, noise suppression, and environmental adaptability must meet rigorous audiological standards without sacrificing the user experience. Miniaturization of high-quality microphones and processors, battery efficiency, and cybersecurity of personal health data are key areas of ongoing development. Moreover, ensuring compatibility across diverse listening environments and individual hearing profiles necessitates sophisticated AI algorithms, potentially involving cloud-based processing and personalized calibration.
Anticipated Innovations and Broader Trends
Looking ahead, integration of biometric sensors—such as heart rate monitors or even neural activity detectors—could elevate these devices from mere hearing aids to comprehensive health monitors. This evolution aligns with emergent trends in digital health: continuous, unobtrusive monitoring, real-time feedback, and remote healthcare integration. Apple’s ecosystem, with its health app integrations and focus on user privacy, positions its earbuds uniquely for such a future.
| Technological Milestones | Projected Development |
|---|---|
| Enhanced AI Personalization | Adaptive hearing profiles tailored to individual environments and preferences |
| Biometric Tracking | Simultaneous health data collection with secure synchronization to health records |
| Extended Battery Life | Battery improvements facilitating all-day health monitoring without frequent recharges |
Conclusion: A Paradigm Shift in Personal Audio and Healthcare
The upcoming AirPods Pro 2 hearing aid update encapsulates a pivotal moment—where consumer electronics morph into vital tools for health management and societal inclusion. As Apple navigates regulatory landscapes and technical challenges, its success could redefine perceptions around hearing health, emphasizing accessibility, discreteness, and proactive care. This convergence embodies a larger societal move towards integrated, wearable health technology that blurs the traditional lines between entertainment, communication, and medical care, promising a future where hearing assistance becomes as seamless and everyday as listening to music.
When is the expected release date for the hearing aid update in AirPods Pro 2?
+While Apple has not officially confirmed a specific date, industry speculation and leaked information suggest a release—or at least a significant firmware update—will occur in the first half of 2024, coinciding with regulatory approvals and product refresh cycles.
Will the new firmware enable full-fledged hearing aid features for everyone?
+The upgrade is likely to introduce advanced sound processing and customization, but full medical-grade functionality may require specific hardware components or FDA clearance, which could limit availability initially or necessitate specific user profiles.
What are the implications for users needing hearing assistance?
+This development could dramatically improve accessibility, offering discreet, affordable, and high-quality auditory enhancement. It also encourages early intervention and continuous health monitoring, fostering proactive ear health management outside traditional clinical settings.
What technical challenges must Apple overcome for successful integration?
+Key challenges include miniaturizing high-fidelity microphones, ensuring energy efficiency for all-day use, maintaining strict audiological standards, and securing sensitive health data against cybersecurity threats.
Could this set a precedent for other tech companies?
+Absolutely. Apple’s move could catalyze a wave of innovation across consumer electronics and healthcare sectors, encouraging regulators, manufacturers, and clinicians to collaborate on next-generation wearable health devices.