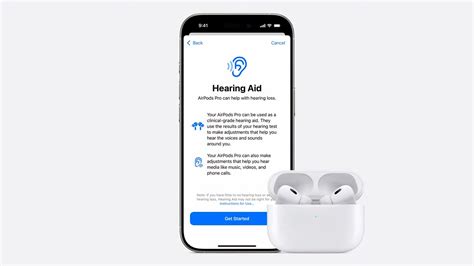
In a world where technological innovation relentlessly transforms daily experiences, the emergence of Apple's AirPods hearing aid feature prompts a fundamental question: Are we witnessing the dawn of a new listening era, or is this merely another step in tech giant’s strategic evolution? The potential of AirPods to double as discreet, high-quality hearing aids could reshape not only how we perceive sound but also how we approach hearing health across diverse populations. This article explores the development timeline, technological advancements, industry implications, and societal impacts surrounding the anticipated release date of AirPods' hearing aid capabilities—an event poised to redefine accessibility and personal audio consumption.
Unpacking the AirPods Hearing Aid Innovation: A Melding of Consumer Electronics and Medical Technology

Apple’s persistent push into integrating health features within consumer devices has culminated in the potential release of AirPods equipped with hearing aid functionality. Historically, hearing aids have been specialized medical devices necessitating complex regulatory approvals, bespoke fittings, and dedicated clinical support. The transition of such features to mainstream consumer earbuds signals a disruptive shift in the hearing health industry. The core question remains: When can consumers expect to access this groundbreaking technology, and what does it entail? To answer this, we must investigate the development timeline, regulatory landscape, and technological intricacies involved.
Historical context and timeline of hearing aid integrations in consumer electronics
For decades, the hearing aid industry operated distinctly from mainstream electronics manufacturers, with devices designed explicitly for auditory assistance. Companies like Phonak, Oticon, and Starkey dominated this space, emphasizing personalized fittings, advanced signal processing, and regulatory compliance. However, recent years have seen tech giants, including Apple, and other consumer electronics firms venture into health and wellness domains, often blurring the lines. The integration of hearing aid features into AirPods traces back to early patents filed by Apple around 2019, signaling an intent to bridge personal audio with health diagnostics.
By mid-2021, whispers from industry insiders suggested Apple was performing extensive testing of this hybrid functionality, with regulatory submissions hinting at imminent launch. The FDA’s (Food and Drug Administration) clearance process, particularly in the US, for over-the-counter (OTC) hearing aids, set the regulatory framework for such devices, influencing Apple’s approach. The key milestones are likely aligned with regulatory approvals, technological refinement, and user safety validation, culminating in a potential release window around late 2023 or early 2024.
| Aspect | Key Developments |
|---|---|
| Regulatory Timeline | FDA OTC hearing aid regulation finalized July 2022; Apple reportedly prepared filings early 2023 |
| Technological Updates | Enhanced noise reduction, spatial audio, and customizable amplification features |
| Market Entry Expectations | Predicted release between Q4 2023 and Q1 2024 based on industry sources |
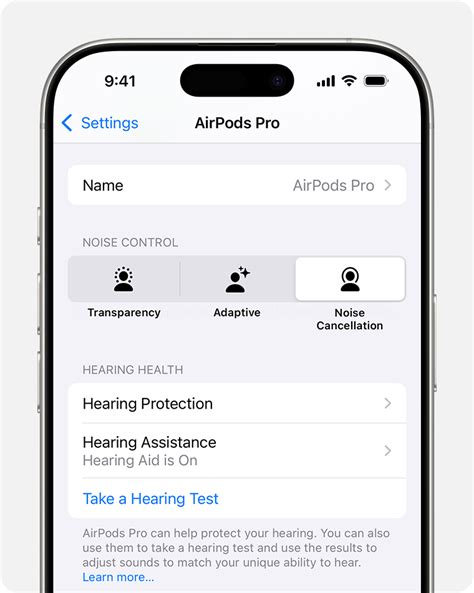
Technological Architecture and User Experience: Inside the Hearing Aid Capabilities of AirPods
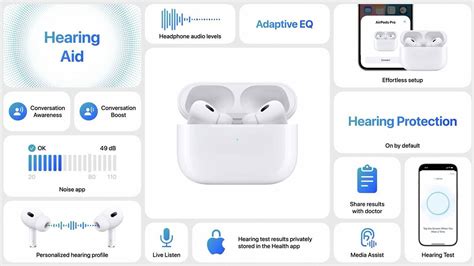
Understanding when the AirPods hearing aid release might occur requires grasping the depths of their underlying technology and how it translates to real-world usability. Apple’s current AirPods Pro and Max models already leverage advanced features such as active noise cancellation, spatial audio, and machine learning-based adaptive sound processing. Extending these features to serve as hearing aids involves significant innovation, including:
- Real-time sound amplification with customizable profile settings for different environments
- Directional microphones capable of focusing on speech signals in noisy backgrounds
- Integration with iOS Health ecosystem for health data collection and user feedback
- Regulatory compliance ensuring safety and efficacy in medical use cases
Particularly, the frequency response range and dynamic sound processing will be critical. Standard hearing aids operate within ranges of approximately 250 Hz to 8000 Hz, tailored to individual audiograms. In contrast, consumer earbuds traditionally prioritize audio fidelity across a broad spectrum but have only recently evolved to include personalized sound profiles. Merging these involves complex DSP (digital signal processing) algorithms that can adaptively filter and amplify sound, offering users a highly personalized listening experience that also addresses hearing impairments.
Regulatory hurdles and safety considerations in deploying hearing aid features in consumer electronics
Ensuring safety remains paramount. Unlike typical consumer devices, hearing aids are subject to stringent medical device regulations, demanding comprehensive clinical trials, evidence-based efficacy demonstration, and ongoing monitoring. Apple’s strategy reportedly involves obtaining FDA approval for OTC usage, a move designed to ease the path for broader consumer access without requiring traditional prescriptions. However, the challenge lies in balancing innovation with adherence to safety standards, as improper amplification or device malfunction could harm users or cause discomfort.
| Relevant Category | Data |
|---|---|
| FDA Approval Status | Expected clearance early 2024, pending clinical validation and safety testing |
| Device Certification | Expected to meet ISO 13485 standards for medical device quality management |
| Consumer Accessibility | Post-approval, predicted availability via standard retail channels and Apple ecosystem |
Implications for Industry, Market, and Society
The anticipated release date of AirPods’ hearing aid functionality not only signals a technological milestone but also heralds broader societal and industry shifts. The advent of accessible, discreet hearing assistance may challenge traditional hearing aid markets, prompting incumbents to innovate rapidly. Simultaneously, it raises questions about digital health equity, data privacy, and regulatory oversight.
Market forecasts and competitive landscape
Industry analysts project a global hearing aid market valued at approximately $10 billion in 2023, with a compound annual growth rate (CAGR) of around 4.5%. The entrance of Apple into this realm, if successful, could capture a significant share due to its extensive user base, brand loyalty, and seamless device integration. Competitors such as Bose, Samsung, and emerging startups are likely to accelerate their own health-oriented audio features in response. The challenge for Apple will be balancing consumer electronics excellence with rigorous medical device standards.
Societal impact and accessibility considerations
One of the transformative potentials of Apple’s move is increased accessibility for individuals with mild to moderate hearing loss who previously relied on costly, clinic-fitted devices. By enabling OTC access via familiar devices like AirPods, Apple could dramatically lower barriers to hearing aid adoption. This democratization extends to remote regions and underserved communities, where traditional services are limited, fostering healthier auditory lifestyles globally.
| Impact Area | Potential Effect |
|---|---|
| Accessibility | Broader reach for hearing aid users, especially in developing regions |
| Market Disruption | Incumbent manufacturers may need to innovate rapidly to remain competitive |
| Data Privacy | Increased health data collection raises concerns about user privacy and data security |
| Regulatory Evolution | Gradual shift toward streamlined approval pathways for health-integrated consumer tech |
Conclusion: The Future Listening Era Begins
The anticipated unveiling of AirPods with hearing aid capabilities marks a pivotal moment at the intersection of consumer technology and healthcare innovation. The precise release date remains a subject of industry speculation, with most informed sources pointing toward late 2023 or early 2024, contingent on regulatory approvals and technological finalization. This development signifies more than just a consumer gadget upgrade; it embodies a vision for accessible, unobtrusive health technology embedded seamlessly into daily life.
As product trials and regulatory processes unfold, it becomes clear that this trajectory aligns with a broader shift toward personalized, AI-driven health solutions accessible directly through familiar devices. The convergence of adaptability, convenience, and medical reliability may soon make hearing aids as common and unobtrusive as wireless earbuds—ushering in a new epoch of auditory experience.
In the end, whether this marks a true revolution in hearing healthcare or simply an evolutionary step depends on execution and oversight. Yet, the potential is undeniable: a future where sound is tailored, hearing is preserved, and technology seamlessly enhances human connection—truly the dawn of a new listening era.